allergy shots vs drops
 Treating Allergies: Are Allergy Shots or Drops Better?
Treating Allergies: Are Allergy Shots or Drops Better?Warning: The NCBI website requires JavaScript to operate. Sublingual immunotherapy Effectiveness in the face of subcutaneous injection immunotherapy in allergic patientsDiego SaportaAsociated in ENT " Allergy, PA 470 North Aveue, Elizabeth, NJ 07208, USAAbstract Although it is generally accepted that subcutaneous injection immunotherapy (SCIT) and sublingual immunotherapy (SLIT) are effective, there is still no significant amount of efficacy. In this work, we performed a retrospective review of the graph and compared the results of the treatment in two groups of patients (both with asthma or asthma-free nasal allergies) who were treated with SCIT or SLIT. Both treatment modalities were of similar effectiveness.1 IntroductionAllergic disease is an increasingly common problem that affects one third of the general population in industrialized countries. Immunotherapy is a treatment modality that can modify the immune response of the affected person so that the affected individual stops reacting to the allergens involved. Immunotherapy is indicated for the treatment of allergic rhinitis (AR) and asthma [], and can prevent the development of asthma in patients with AR [, ].Immunotherapy can be given by different routes between which we find injectable and oral vaccines. Injectable vaccines refer to classical subcutaneous injection immunotherapy (SCIT) generally known as "allergy injections". Oral vaccines refer to sublingual immunotherapy (SLIT) where allergens are given as drops to the sublingual area, although the term oral vaccines may also include allergy tablets []. The aim of this study is to compare the effectiveness of the treatment results in patients with nasal allergies, with or without asthma, which were treated with either of these two treatment modalities: - What? There is a bulky body of scientific evidence that demonstrates that these two treatment modalities are effective in managing allergic conditions, but the issue of these two modalities that have similar effectiveness has not yet been fully addressed. A literature review reveals only a few articles that directly address this issue [–]. In five of these reports [–] it is considered that SCIT and SLIT are equally effective. In a report [] SCIT is found to have better results, and a report [] finds both equal to effective for AR patients but SCIT most effective for asthma patients. In our own experience, SLIT and SCIT seem to be of similar effectiveness [] This report will compare the effectiveness of one against the other. SCIT is a well-established treatment modality that has been successfully used for many decades and is relatively well tolerated. From time to time, patients can develop severe reactions that can rarely result in mortality []. SLIT is also a very old treatment modality (the earliest description is 1900) and yet, although it is commonly used in Europe, it is still not well established in the USA []. Over the past 20 years the European medical community produced a lot of high quality tests that suggest SLIT is safer than SCIT [, ]. Although no case of mortality has been reported with SLIT [, ] this is not the case with SCIT [, ]. SLIT is so safe and easy to administer that patients are treated at home [].2. Methods This study constitutes a retrospective and consecutive review of allergy patients treated by the author in his private office. An alphabetically revised diagrams of active patients to determine eligibility. The inclusion criteria were as follows: a patient of any age with nasal allergies with or without asthma that was treated with immunotherapy for at least 6 months and had at least 2 full evaluations. A complete evaluation involves the score of symptoms, the evaluation of the use of drugs and the determination of the value of the maximum flow meter (MCF). These evaluations are carried out every 3-6 months as treatment progresses. Because evaluations depend on patient cooperation, not all patients had the same number of evaluations, but any patient who was considered a candidate had to have at least 2 evaluations. We compared the first evaluation (pretreatment) and the last evaluation that the patient had right at the time of the study inclusion. These assessments were considered pre-treatment and post-treatment. Symptoms in pretreatment evaluation and the amount of medication the patient was taking at that time reflect how the patient was doing without treatment of immunotherapy. Ethical considerations. The privacy of the subjects was respected through the collection and registration of data so that the subjects could not be identified, directly or indirectly, through identifiers linked to the subject. In other words, a patient's confidentiality would be protected by entering data into a simple spreadsheet with non-specific identifiers such as patient number 1, patient not. 2, and so on with later re-enviation of the patient's chart, according to usual procedure. The content of the distribution sheet was made anonymous and ready for statistical analysis. 2.1. Use Decision SCIT or SLITAfter discussing with the patient about their allergies and advising on environmental modification manoeuvres a discussion on treatment options including immunotherapy follows. In our SCIT or SLIT office it is used to treat patients with inhalant allergies with or without bronchial involvement. The decision to use one or the other is sometimes taken by the patient, sometimes advised by the treating doctor. Economic considerations, which live away from the office, busy schedule, or "phobia need", are examples of when a patient can choose SLIT. Having severe asthma, being a very young patient or having medical problems that can make the SCIT administration risky are examples of why the doctor you treat will advise you SLIT.2.2. Test and Treatment Management All patients were tested using an intradermal dilution skin test five times (IDT) as AAOA teaches [, ]. The test includes several panels: dust, dander, epidermals, moulds and pollen for our geographical area (). Table 1 Allergy Test Panels. Polvo, dander. and epidermalesMoldsTreesGrassesWeedsMite pteronyssinusAlternariaAshBermudaCockleburMite farinaeAspergillusBeechJohnson PlantainDogCladosporiumBirchTimothyGoldenrodCatCurvularia Elder Box Lambs Quarters AmericanRoachEpicoccumElm PigweedRoach germanicaFusariumHickory Ragweed Helminthosporium Roble Sagebrush MucorSycamore Sheep Sorrel Penicillium Pullularia Standardized antigens were used for testing and treatment when available; otherwise we used extracts of weight/volume antigen []. After identifying the minimally reactive concentration of the antigen (which means the first reactive serum) for each of the patient's reactive allergens, the SCIT bottles or the SLIT bottles were formulated including all positive results (reactive allergens in the intradermal examination) in the treatment mixture. Patients at SCIT were treated according to AAOA guidelines [, ]. Patients in SLIT were treated according to a previously published protocol [] where the dose slowly progresses from 1 drop per day to 5 drops per day until reaching the most concentrated mix in the SLIT bottle. The formulation is the same for injectable and oral vaccines. 2.3. Amount of antigen deliveredWhile the antigen concentration is exactly the same for SCIT and SLIT, but SLIT is administered daily [], patients in SLIT will receive a greater amount of antigen each week than those treated with SCIT. Injectable vials are mixed with a volume of 5.0 mL. The SLIT bottles are mixed with 7.5 mL. If we consider a single allergen, for example, Dermatophagoides pteronyssinus (DP), standardized dust mite DP has a concentration of 10,000 AU/mL containing 68 mcg/mL of Der p 1 and 71 mcg/mL of Der p 2 antigens []. If the concentration of minimally reactive antigen occurred in dilution no. 3 and the dosage was advanced until a bottle of the manufacturer's concentrate was mixed, the cumulative dose that this patient would receive weekly by SCIT would be 200 UA per week, while a patient treated by SLIT would receive 464 UA per week []. As indicated above, the initial concentration of allergens in SCIT and SLIT is the same: 80 AU/mL that in both circumstances the extract (with 10,000 AU/mL) will be diluted 125 times. After one year of treatment the patient in SCIT would receive 9680 AU and the patient treated by SLIT would receive 21149 AU or 2.18 times more allergen [].2.4. Sample comparison in reference to allergen Reactivity A chi-quarter test was applied for the following allergens: dust mite, cat, cockroach, mold, tree chicks, grass chicks and grass chicks for both groups, SCIT and SLIT.2.5. Diagnosis of asthmaThe diagnosis of asthma was based on the presence of recurrent cough, chest stiffness, SOB or sybilance [], having a spirometry consistent with the obstruction of airflow or having the symptoms respond to the administration of a short-acting broncoagonist (SABA). 2.6. ScotRegistered symptoms include spongy nose, sneezing, nasal obstruction, spicy eyes, ear itching, coughing, shortcircuit and wheezing. These were marked according to Fell's method [] with a numerical analogue of 0 to 3 as follows:0 = non-present symptom,1 = symptom is mild,2 = symptom is moderate,3 = symptom is severe. The use of medicines was also evaluated on a similar numerical scale as follows:0 = medication is not being used1, = medication is being used once a week or less.2 = medication is being used 2-3 times per week,3 = medication is being used 4 or more times per week. Medicines were grouped generically as allergy pills, intranasal steroids (INSs), and short-acting broncho-agonists (SABAs) in the case of asthmatic patients. The value of the PFM determination was used as a parameter to be recorded in the meeting of each patient.3. ResultsNinety-three graphics met the inclusion criteria, 50 in SCIT and 43 in SLIT. Among the 50 patients in SCIT, 20 (40%) were men, 30 (60%) women who ranged from 2.33 to 75 years old (average 45 ± 17.8 SD). This compared to 43 patients in SLIT of whom 21 (49%) were male, 22 (51%) females ranging from 1.66 to 75 years old (average 35 ± 20.8 SD). There are no statistical differences between the demographics of both groups. The analysis of the covariance of the dependent variables for which significant effects of pre/postage interaction were obtained by treatment modality did not reveal gender or age to take into account an important variable variation dependent; in other words, the results were not affected by age or gender, so that both groups can be considered homogeneous. Both groups were also compared with the results of the tests. A chi-quarter test was applied for the following allergens: dust mite, cat, cockroach, mold, tree chicks, grass chicks and grass chicks. Results indicate that there are no statistical differences between the two groups (in P There were 3 children For all patients, pre- and post-treatment averages were statistically compared for each symptom, the use of drugs and PF value through repeated measurement analysis of variance (ANOVA). The results of the two treatment modalities (SCIT versus SLIT) were also compared to the factor between ANOVA subject matter (). The same tests were completed for the use of medicines (). For the evaluation of the PF, the pre- and post-treatment values were compared (). Table 2 Symptom ResultsNo. of patients Before (medium)After (medium) Value P of t-test Meaning of SCIT/SLIT × before/after the interaction Runny nose SCIT472.10.7Table 3 Use of medicines.Medicationno. of patients Before (medium)After (medium) P-value of t-test Meaning of SCIT/SLIT × before/after interaction Pills in SCIT372.00.5Table 4Peak Flow Tests. (L/m = liters per minute). PFM (L/m)no. of patients Before (medium) T-test value Meaning SCIT/SLIT × before/after the PFM interaction at SCIT443684673.1. Symptom Results In the average value for each symptoms score before treatment and at the time of data collection is shown for both treatment modalities. The result of the significance test is shown for each symptom within each treatment modality (t golden test). Finally, the result of the statistical analysis is shown to compare the improvement of symptoms to one or the other treatment modality. All symptoms had a significant improvement with both treatment modalities. The lack of breath and sybilance had significant improvements in P Wheezing and cough were the only symptoms that seemed to respond better to any SCIT (with slightly better cough, P = 0.037) or SLIT (with a little better, P = 0.0024), although both symptoms significantly improved regardless of the treatment modality. For the remaining symptoms there was no significant difference between both treatment modalities. 3.2. Results of drug use Both SCIT and SLIT provided an equally significant reduction in drug use (P 3.3. Results of changes in PFM Value PF values prior to treatment and at the time of the last patient assessment are shown in . Both treatment modalities were equally effective in achieving a significant increase in PF values (P 4. Discussion This document is a retrospective review of the chart and therefore lacks the rigor of a randomized prospective study with a placebo control group that is very difficult to do in a private office environment. While a covariance analysis is useful, it is not a perfect solution. A large-scale future study should be planned to include previous design features. We note that patients usually come to the office already using one or more allergy medications. This study, like others, shows that immunotherapy, whether SCIT or SLIT, will lead to reduced use of AR and/or asthma medications. It was not the purpose of this document to evaluate the effect of medicines on allergy symptoms, but to compare the effects of SCIT versus SLIT on the use of medicines. Both treatment modalities led to the reduction of antihistamines, inhaled nasal steroids and SABAs. The slight imbalances in the demographic characteristics of the groups on SCIT and SLIT were not statistically significant and did not affect statistical results. The reason there are more young patients and more asthmatic patients in the SLIT group can be explained by the fact that SLIT is safer and easier to manage so it is suggested more frequently for these patients difficult to administer. In fact, we would have expected a much more pronounced difference; but less than expected SLIT chose because it is not covered by insurance. Patients at SCIT have been treated for a longer period of time because SLIT was added to our practice later than SCIT. The improvement of the asthmatic symptoms of sybilance and SOB and the decrease in the use of SABA were significant in P The advantage for SCIT in the treatment of cough is real, but the effect size (eta-squared) is only 0.025, which means that it only represents 2.5% of the variance in the pre- versus post-treatment differences, which is not much. It can therefore be concluded that SCIT and SLIT exhibit similar effectiveness. The advantage of SLIT in the treatment of sybilance may have been influenced by our own bias of suggesting the use of SLIT to asthmatic patients as a safer treatment modality. Therefore, it is more likely that patients with higher symptoms scores were present in the SLIT group. Our findings show that SLIT is not only effective in the control of symptoms in patients with nasal allergy with or without asthma, in the decrease of the use of medicines in such patients, and in the improvement of the parameters of lung function, but it also seems that SLIT is as effective as SCITThese findings are in accordance with those published in European literature [, ] but this presentation does not doubt lack the scientific validity of other controlled reports []. If SLIT became a treatment modality approved by the FDA (and hopefully) reimbursed by insurance companies many more patients could be receptive to immunotherapy which is a treatment capable of altering the immune mechanisms responsible for the development of allergic conditions []. PF values for asthma control should be taken as a guideline only because the predicted lung function has a high degree of variability with significant differences in PF values according to the presence or not of lung disease, smoking, age, sex and even the patient's social environment [–]. Having the advantage of providing results quickly, and requiring little training (patient and technical staff), the PFM device is useful to monitor progress during immunotherapy []. It is very useful when changes in PF values are compared to the initial value of each patient, registered at the time of initiation of the treatment []. For the purposes of this study, individual improvement with therapy is not reported, but rather a general trend, so the use of PFM provides a gross indicator of change. Immunotherapy is given for a long period of time. Some of our patients were children, and are expected to grow during treatment. Certainly, the use of a PFM as a tool to determine the improvement of lung function adds uncertainty about whether the improvement of PF value is related to clinical improvement or patient growth during treatment. In this study the number of young patients was not large. On the other hand we have shown that the value of PF in patients treated by immunotherapy increases regardless of age or asthmatic condition []. In our experience, the use of SLIT with multiple antigens has allowed us to treat patients who otherwise would not have received immunotherapy, or would not have continued to receive immunotherapy, such as asthma patients with poorly controlled asthma, patients with severe arm reactions, very young patients who are difficult to administer vaccines or patients whose schedules prevent them from being compatible.5. Conclusions These results suggest that SCIT and SLIT exhibit similar effectiveness. SLIT objectively improves symptoms scores for asthma and AR while decreasing the use of allergy medications and SABAs. Given the increased risk and difficulty in treating asthmatic and young patients, these results suggest that SLIT should be considered as the main treatment modality for these patients, considering SCIT only for treatment failures. The results of this study are in accordance with European literature and would therefore support the inclusion of SLIT in the routine management of allergic disease.RecognitionStatistical analysis carried out by Jeffrey S. Kane, Ph.D., Professional Statistics Services. Yassir M. Samra, Ph.D., Associate Professor of Management, conducted a review of previous statistical analysis and analysis of "Compare samples in reference to allergen reactivity". ReferencesFormats: Share , 8600 Rockville Pike, Bethesda MD, 20894 USA

Allergy Drops vs Shots | Advanced ENT & Allergy Center

Pros and Cons of Allergy Shots | Carolina Asthma and Allergy, Charlotte

Treating Allergies: Are Allergy Shots or Drops Better?

Treating Allergies: Are Allergy Shots or Drops Better?

Treating Allergies: Are Allergy Shots or Drops Better?
Immunotherapy

Allergy Drops - Aspire Allergy & Sinus

Treating Allergies: Are Allergy Shots or Drops Better?

Injection & Sublingual Immunotherapy | Jason Sigmon, MD, FAAOA

Allergy Drops: What to Know About Sublingual Immunotherapy

Allergy Drops vs Allergy Shots - Which is Better?

Should I use Allergy Shots or Allergy Drops?
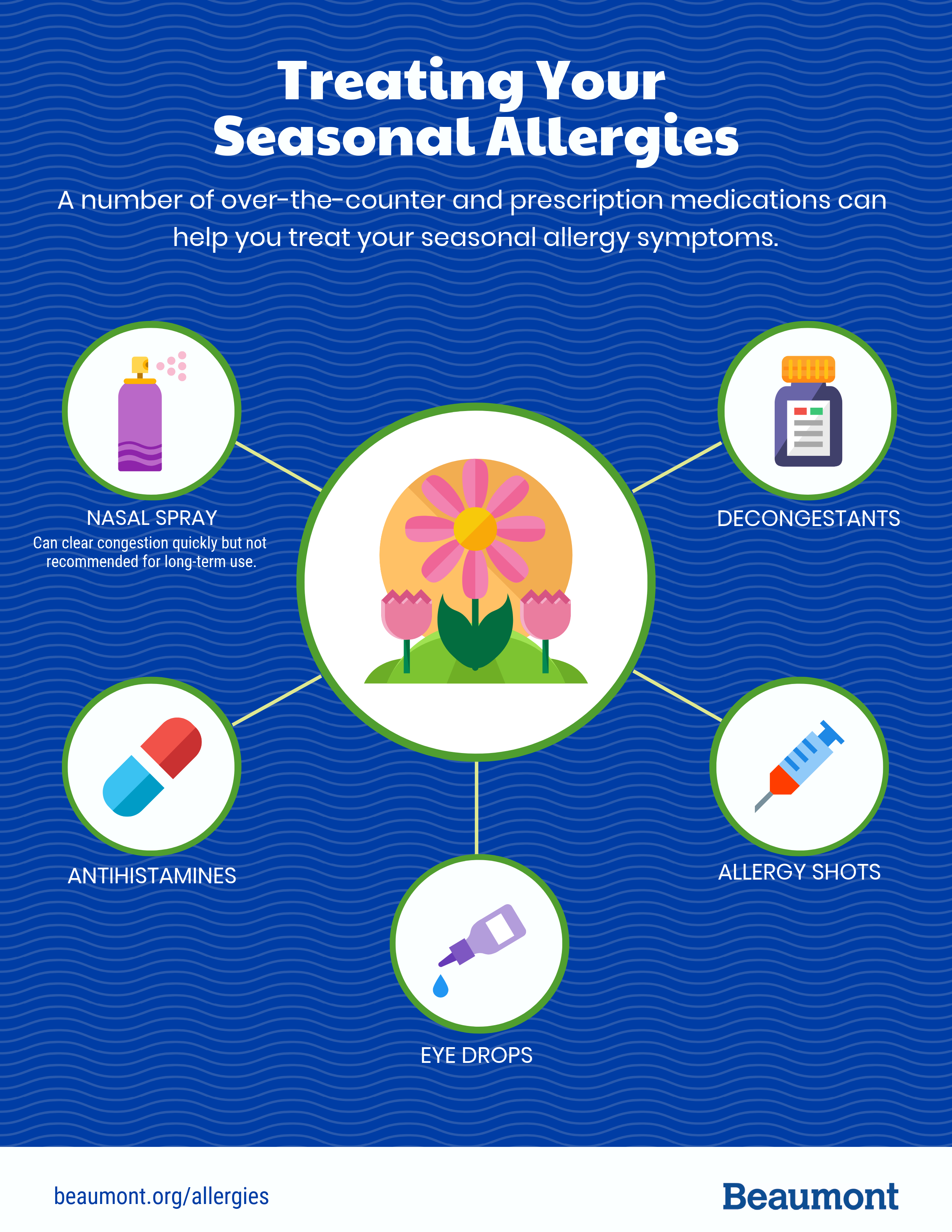
Treating Seasonal Allergies | Which Medications and When | Beaumont Health
Allergy shots (immunotherapy): Efficacy, side effects, and types
Allergy Immunotherapy: Relief that is Nothing to Sneeze at | BioSpace

Treating Allergies With Pills or Drops Instead of Shots - The New York Times

Treating Allergies: Are Allergy Shots or Drops Better?

Best Allergy Treatment: Shots vs. Drops - Which is the best medication for you? : Sinus & Allergy Wellness Center: Otolaryngology

Allergy Shots vs. Allergy Drops - Hedberg Allergy

We offer Allergy Drops instead of shots - TRI-Valley Pediatrics Inc - Pediatrics for Family Health

Why I Prefer Allergy Shots vs Allergy Drops (Which Treatment is Best)
Allergy Shots: Side Effects, Efficacy, Cost, and What to Expect
![infographic] Allergy-Induced Asthma infographic] Allergy-Induced Asthma](https://www.allergyeasy.com/wp-content/uploads/2015/04/2015-4_Allergy%E2%80%90Induced-Asthma.jpg)
infographic] Allergy-Induced Asthma

Treating Allergies: Are Allergy Shots or Drops Better?
Pet Allergies | Allergy Shots Versus Allergy Drops for Pets | PetMD

Allergies | Midwest ENT Centre | St. Peters, MO

Allergy Drops vs Allergy Shots | Sinus & Snoring Specialists
Allergy Treatment - allergy shots and allergy drops for allergies

New study compares allergy shots vs. drops - WRCBtv.com | Chattanooga News, Weather & Sports

Why I Prefer Allergy Shots vs Allergy Drops (Which Treatment is Best)
Hate Allergy Shots? Oral Allergy Drops are a Pretty Good Option for Some Allergy and Allergic Asthma Sufferers, Study Review Shows - 03/26/2013

Allergy Drops vs. Allergy Shots - Columbia Allergy and Asthma Specialists

Allergy Drops vs. Allergy Shots: What's Best for You? | Body + Mind

Allergy Shots vs Allergy Drops | Allergy Relief, Cure | Colorado

Allergy Shots vs Drops | Scottsdale Sinus & Allergy
![infographic] Why Choose Sublingual Immunotherapy infographic] Why Choose Sublingual Immunotherapy](https://www.allergyeasy.com/wp-content/uploads/2014/12/2014-11.jpg)
infographic] Why Choose Sublingual Immunotherapy

AllergyEasy Introduces Sublingual Immunotherapy Drops for Oral Allergy Syndrome | SnackSafely.com
A Guide to Sublingual Immunotherapy Allergy Shots vs Allergy Drops

How effective is immunotherapy (allergy shots or drops)? | Allergy Partners

Allergy Drops vs Shots | Advanced ENT & Allergy Center
Posting Komentar untuk "allergy shots vs drops"